A special post
A big moment for me as a scientist!
7/4/20243 min read
This is no ordinary blog post - I have a big announcement! This week we published a research paper which describes years of hard work applying computational modelling to genetic sequence data which has resulted in our ability to predict patient prognosis across multiple cohorts of lymphoma patients.
If there is one thing that feels better than making a scientific discovery then maybe it is sharing that discovery with the world. Science is necessarily collaborative and a team effort and we are always standing on the shoulders of giants, but I am also proud of what we have done here and I hope that it is useful for other scientists going forward.
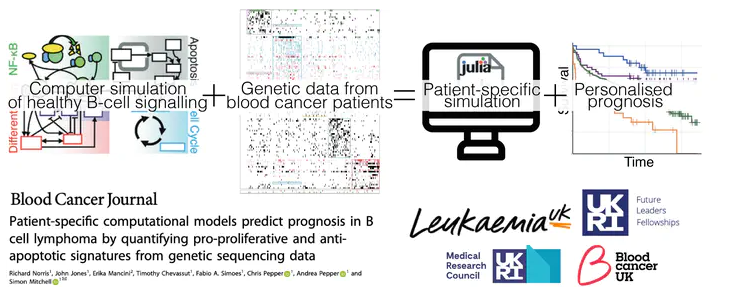
So far I haven't talked in much detail about what I do and the projects I work on so I will try to explain what this paper describes and why we (everyone who worked on it) believe it is important.
Disclaimer: unlike my other blog posts so far where I've tried to limit the number of 'big concepts' that I try to explain, but I'm not going to put such limits on this one, so apologies if it seems like a lot.
When we talk about types of cancer (breast, prostate, lung, etc etc) it sounds like there is little to distinguish patients suffering from them. But this could not be further from the truth. In fact, in some ways, every single cancer (and I mean every cancer patient, even within the same tumour type) is different. We call this heterogeneity. Despite this, all too often, a on-size-fits-all treatment is given to every patient with the same type of tumour. As you might expect, some patients respond well to the treatment and others do not, meaning their cancer will eventually come back, if it ever went in the first place.
What we, and others, have been trying to do for many years is to identify the differences between patients with the same type of tumour so that we can design therapeutic approaches in a more personalised way. Due to amazing advances in technology we can now (fairly cheaply and easily) sequence DNA from cells in a patient's cancer and, by comparing patient DNA to ('normal') DNA from non-cancerous cells, identify the genetic changes (or mutations) driving the cancer. Previously scientists have applied clever algorithms to group cancer patients according these genetic changes. While this approach is useful, the effect of these mutations on the complex signalling pathways controlling various processes within cancer cells is largely ignored.
What we have done is to use a model in which many of the reactions that are part of these signalling pathways are represented as mathematical equations, made possible by decades of research in cell biology. We can then convert these equations into something that a computer can understand, meaning that we essentially create virtual cells. I'm not going to go into exactly how this works here as it would take too long, but feel free to reach out if you want to ask something.
Importantly, each of the equations has one or more parameters that control things like how much of a particular protein is produced or degraded. These parameters can be changed to reflect changes in the regulation of each process, which represents mutations in DNA.
We then had to work out which mutations corresponded to which parameters in the model and we created a kind of dictionary which allows the computer to read this information and then create a patient-specific model by modifying the parameters according to mutations present in individual patients. This means that once you set the code running, you can simulate cancer cells from hundreds of different patients.
Given that the most common mutations in B cell lymphomas affect how quickly a cell dies (apoptosis) and proliferates (cell division), as discussed in the first part of the paper, we decided to quantify the levels of the most downstream components of the apoptosis (cSmac and cCytoC) and cell cycle (Cdh1) pathways in the model. From this we split the patients into groups based on the abundance of these components.
We found that simulations of patient mutations that showed concurrent up-regulation (higher levels of cSmac and cCytoC and Cdh1) of cell cycle and apoptosis pathways had the worst prognosis following standard treatment (multi-drug chemotherapy). This pattern was consistent across multiple groups of lymphoma patients.
So...
Simulations can reveal when mutations combine in unexpected ways.
By simulating individual patients we can predict when mutations will combine to create aggressive tumours.
Questions:
If we want to design clinical trials that succeed should we target these patients?
Perhaps we can give kinder/reduced treatments to the good prognosis patients?
What’s next?
Now that we can identify patients who would benefit from alternative treatments, can we use these virtual patients to find targeted therapies that will work?
Could this work in other diseases? Myeloma? CLL? or even breast cancer?
As I said, this is a lot to write about in one post, but I hope it gives a useful summary of our work and makes it a bit more accessible, because what's the point of doing science if you can't tell people about it?